Home › Treatment › Operations › LVAD
Intended for patients with end stage heart failure
 Heart failure patient with an LVAD zip-lining in Costa Rica
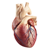
Heart failure patient with an LVAD zip-lining in Costa RicaCurrently, about 5.1 million Americans have heart failure, with 825,000 new cases diagnosed every year. Although there are many different methods to treat the condition, heart transplant may be the best treatment for patients with end-stage heart failure, who are able to tolerate immune system suppression medications. Unfortunately, there are less than 3,000 donor organs available worldwide per year. Due to such limiting factors, scientists have developed numerous ways to optimally support failing hearts (with medication and/or devices), so that sufferers’ lives may be improved, and in some cases extended. Search for an optimal circulatory-support device began in 1964, with the National Institutes of Health (NIH) artificial-heart program. In 1994, the Food and Drug Administration (FDA) approved a pneumatically driven LVAD (pronounced “l-vad”) as a bridge to transplant (until a matching organ donor is available). Four years later in 1998, the FDA approved a self-contained, vented electric device to replace the pneumatic one that allowed patients to go home while awaiting heart transplant. This technology was later refined into a quieter and smaller device, HeartMate II. Even so, the overall mortality rate due to end-stage heart failure remains high, mainly due to the limited number of donor heart transplant organs. LVAD is also used as a long-term support destination therapy for patients who are not candidates for heart transplant. The HeartMate II is one of several LVAD devices. It is designed to last longer with the simplicity of only one moving part. It is also much lighter and quieter than its predecessors. One of the major differences is the rotary action which creates a constant flow of blood instead of the pumping action created by its predecessors.
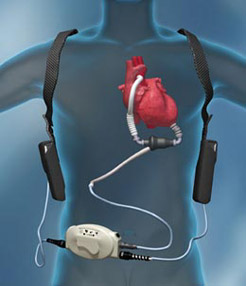
So, how does an LVAD work? It has three parts: a rotor suspended by a magnet, an electronic controller, and a power supply. The pump is about 3 inches in length and weighs approximately 10 ounces, made of titanium with a biological friendly lining. Placed in the abdominal cavity, the LVAD takes blood from the left ventricle and pumps it into the aorta.Two external batteries, via a cable through the abdomen, supply the power for this pump. They are carried in underarm holsters or a waist pack. The electrical controller is a small computer like component that adjusts functions of the pump, such as rotor speed. You can find more details of how an LVAD works at the site www.thoratec.com This device pumps blood through the body when the bodies heart is not strong enough to adequately do it by itself. The LVAD is designed to assist the heart in pumping blood, therefore patients implanted with an LVAD will often have a very faint pulse due to the LVAD’s constant flow provided by the impeller.


 Español
Español